|
Alzheimer's Basics
Introduction
Alzheimer's disease (AD) is
a degenerative disease of the brain from which there's no recovery.
The disease slowly attacks
nerve cells in all parts of the cortex of the brain & some surrounding structures, thereby impairing a person's abilities
to:
-
-
-
coordinate movement
-
remember
Ultimately, a person
with AD loses all memory & mental functioning.
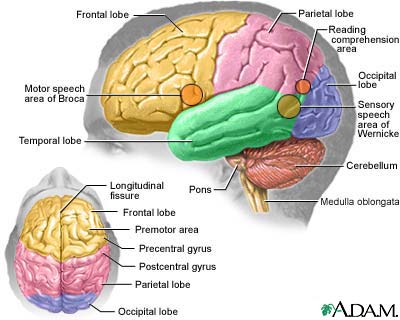 
Causes
Researchers are
finding specific biologic factors involved with Alzheimer's disease. Various environmental & genetic players appear to
contribute to or trigger the process by which these factors destroy nerve cells leading to this disease.
Biologic Factors in the Brain
Imaging techniques in
patients with Alzheimer's disease have found significant loss of cells & volume in the regions of the brain devoted to
memory & higher mental functioning.
Important
abnormalities have specifically been observed during biopsies:
- Twisted nerve cell fibers, known as neurofibrillary tangles
- A sticky protein called beta amyloid
Other factors also play a role.
  The Effects of Neurofibrillary
Tangles & Beta Amyloid in Alzheimer's Disease. These biologic factors appear to be involved in the development
Alzheimer's disease in the following ways:
- Neurofibrillary tangles are the damaged remains of microtubules, the support structure that allows the flow of nutrients thru the neurons
(nerve cells).
A
key component in these tangled fibers is an abnormal form of the tau protein, which in its healthy version helps in
the assembly of the microtubule structure. The defective tau, however, appears to block the actions of the normal version.
- Beta Amyloid
(also called A beta) is the 2nd significant finding.
This insoluble protein accumulates & forms sticky patches called neuritic plaque, which are found surrounded by the debris
of dying nerve cells in the brains of Alzheimer's victims.
- Amyloid precursor protein (APP)
is a large nerve-protecting protein that's the source of beta amyloid.
In
Alzheimer's certain enzymes, particularly those called gamma-secretases, snip APP into beta amyloid pieces. This process
is controlled by factors called presenilin proteins. (Genetic abnormalities that affect
either APP or presenilin proteins occur in some inherited cases of early-onset Alzheimer's.)
- High levels of beta amyloid are
associated with reduced levels of the neurotransmitter acetylcholine. (Neurotransmitters
are chemical messengers in the brain.) Acetylcholine is part of the
cholinergic system, which is essential for memory & learning & is progressively destroyed in Alzheimer's disease.
- Beta amyloid may also
disrupt channels that carry sodium, potassium & calcium. These elements serve the brain as ions, producing electric charges
that must fire regularly in order for signals to pass from one nerve cell to another.
If the channels that carry ions are damaged, an imbalance can interfere with nerve function & signal transmission.

Other Proteins.
Researchers have now identified other important proteins in the areas of the brain affected by
Alzheimer's disease.
- ERAB (endoplasmic-reticulum
associated binding protein) appears to combine with beta amyloid, which in turn attracts new beta amyloid
from outside the cells. High amounts of ERAB may also enhance the nerve-destructive power of beta amyloid.
- AMY plaques resemble beta amyloid so closely that researchers
were able to detect them only with the use of highly sophisticated techniques.
- Elevated levels of a protein called prostate apoptosis response-4
(Par-4) may cause nerve cells to self-destruct.
Oxidation & the Inflammatory Response
Researchers are also attempting
to discover why beta amyloid is so toxic to nerve cells. Some researchers are focusing on 2 processes in the body that may
be involved with Alzheimer's disease:
- oxidation
- inflammatory process
There's some evidence that
such events can begin decades before Alzheimer's disease actually develops. One scenario for their role in Alzheimer's is
as follows:
The Role of Oxidation.
- As beta amyloid breaks down it releases unstable chemicals
called oxygen-free radicals. Once released, oxygen-free radicals bind to other molecules thru a process called oxidation.
- Oxidation is the result of
many common chemical processes in the body, but when oxidants are overproduced, they can cause severe damage in cells &
tissue, including even affecting genetic material in cells (its DNA).
Oxidation is known to play
a role in many serious diseases, including coronary artery disease & cancers & experts believe it may also contribute to Alzheimer's.
The Inflammatory Response.
- One result of oxidation is the marshaling of immune factors
to repair the cellular injuries it produces. Overproduction of some of these factors, however, produces the so-called inflammatory response, in which the immune process itself can actually damage the body's
own cells themselves.
- Principle immune cells in the brain are called macrophage/microglia
(M phi). In the healthy brain, they play an important protective role against invading organisms.
However, when they're activated
by beta amyloid oxidation, they release toxic molecules called cytokines, which are known to cause harm.
i.e., significantly high levels of interleukin-6, a specific
cytokine, have been detected in people with Alzheimer's.
- Other inflammatory factors of specific interest in Alzheimer's
research are the enzyme cyclooxygenase (COX) & its products called prostaglandins.
Excess amounts of these factors
may increase levels of glutamate. Glutamate is an amino acid that excites nerves & when overproduced, is a powerful
nerve-cell killer.
- The inflammatory process has also been associated with the
release of soluble toxins called amyloid beta derived diffusible ligands, which some investigators believe may prove to key
players in the destructive process.
Genetic Factors
Major research targets in
Alzheimer's disease are the factors responsible for beta amyloid build-up & concentration in certain people & not
in others.
Genetic factors are believed
to play a role in many cases. In 2003, the National Institute on Aging (NIA) launched the ambitious AD Genetics Initiative,
a 3-year national project to bank genetic material from families who have at least two members with late-onset Alzheimer's.
The ApoE Gene & Late-Onset
Alzheimer's. The major target in genetic research on late-onset Alzheimer's disease (called LOAD) has been
apolipoprotein E (ApoE), which plays a role in the movement & distribution of cholesterol for repairing nerve cells during
development & after injury.
 The gene for ApoE comes in three major types:
- ApoE4. Studies have reported the greatest
deposits of beta amyloid in people with ApoE4, which is now believed to be a major risk factor for late-onset Alzheimer's.
Some evidence suggests that the ApoE protein removes beta amyloid but the ApoE4 variant does so less efficiently than other
ApoE types. (ApoE4 has also been studied for years as a risk factor for heart disease.)
- ApoE3 and ApoE2. Fewer beta amyloid deposits
have been observed in people with the ApoE3, and the fewest deposits have been observed in people with ApoE2, which may actually
be protective.
People inherit a copy of one type from each parent, but Alzheimer's
disease is not inevitable even in people with two copies of the ApoE4 gene. Reports vary widely in estimating the extent of
risk:
- People without ApoE4 have an estimated risk of between 9 -
20% for developing Alzheimer's by age 85.
- In people with one copy of the gene, the risk is between 25
- 60%.
- In people with two copies, the risk ranges from 50 - 90%. (Only
2% of the population carries two copies of the ApoE4 gene.)
Some researchers suspect that some specific variation of the
ApoE4 gene or combinations with other genes are critical for the disease, since many people who carry the ApoE4 exhibit no
signs of Alzheimer's. For example, evidence suggests that genetic factors play a role in a common subtype of late-onset Alzheimer's
disease that also includes psychosis. An important 2002 genetic study has identified certain genetic linkages associated with
ApoE4 that appear to play a strong role in this subtype.
Other Genetic Factors in Late-Onset Alzheimer's.
Most people with late-onset Alzheimer's disease do not carry the ApoE4 gene. Increasingly, researchers believe that many cases
of late-onset Alzheimer's are a result of a collaboration of genetic factors that participate in the process of producing
or degrading beta amyloid. Some under investigation are the following:
- Researchers are now targeting chromosomes 9, 10, and 12 as
possible locations for genetic factors involved with Alzheimer's disease. (The ApoE4 gene is on chromosome 19.) In 2005, researchers
announced that mutations linked to the ubiquilin 1 (UBQLN1) gene, located on chromosome 9, might be associated with increased
risk for late-onset Alzheimer's disease.
- Researchers have detected mutations in the proteins amyloid
precursor protein (APP) and ubiquitin-B (Ubi-B), which may account for some cases of late- and early-onset Alzheimer's. Such
mutations are not inherited, however, but appear to be genetic mistakes that occur during transcription, the coding process
in which DNA establishes the pattern for the production of its proteins and other molecules.
Genetic Factors for Early-Onset Alzheimer's.
Scientists are coming closer to identifying defective genes responsible for early-onset Alzheimer's, an uncommon, but extremely
aggressive form of the disease.
- Mutations in genes known as presenilin-1 (PS1) and presenelin-2
(PS2) account for most cases of early-onset inherited Alzheimer's disease. The defective genes appear to accelerate beta amyloid
plaque formation and apoptosis, a natural process by which cells self-destruct.
- Genetic mutations in the genes that control amyloid precursor
protein (APP) are also being targeted as causes of early-onset Alzheimer's. The genetic disease Down syndrome, for example,
overproduces beta-amyloid precursor protein (APP), the source of beta amyloid, and almost always leads to early Alzheimer's.
Other APP mutations are being identified.

Environmental Factors
Researchers are also investigating environmental factors (infections,
metals, industrial and other toxins) that may trigger oxidation, inflammation, and the disease process, particularly in people
with a genetic susceptibility to Alzheimer's.
Infectious Organisms. Slow, infectious
viruses cause a number of other degenerative neurologic diseases, such as kuru and Creutzfeldt-Jakob disease.
Although no specific virus has been linked to Alzheimer's, some
researchers theorize that people with a genetic susceptibility to Alzheimer's may be vulnerable to the actions of certain
viruses, particularly under circumstances when the immune system may be weakened.
Metals. Some laboratory studies have reported
excessive amounts of metal ions such as zinc, copper in the brain of people with Alzheimer's disease. Such ions may possibly
change the chemical architecture of normal beta amyloid, making it more harmful. A mildly acidic environment appears to be
important in the process that binds these metals to beta amyloid. Experts observe that such conditions (acidic environment
and higher levels of zinc and copper) commonly occur as part of the inflammatory response to local injury.
Electromagnetic Fields. Some studies on
people exposed to intense electromagnetic fields (EMF) have reported a higher incidence of Alzheimer's. However, the association
between EMF and Alzheimer's is very weak.
Family Support System for Alzheimer’s
Behavior Problems
by Dr. Roeltgen's Blog
Friday, February
16th, 2007
What can a
family support system do for behavioral disturbances in someone with Alzheimer’s disease?
Approximately 50 to 80% of persons diagnosed with Alzheimer’s disease have some type of behavioral or
psychiatric condition, such as:
- agitation: restlessness, irritability, resistiveness or a combination of the 3 & may include problems such as
wandering
- psychosis: a disturbance in the perception of reality & may be represented by abnormal thoughts or hallucinations.
- disinhibition: a loss of social restraint & may include aggression
or sexual suggestiveness.
- a combination of these
Although loss of memory &
loss of abilities is a devastating part of this disease, the behavioral disturbances may have a greater impact on caregivers.
These disturbances are difficult
to manage & not always easily treatable with medication & therefore they can put great stress on caregivers.
Consequences may include caregiver
burnout or illness, patient abuse & institutionalization sooner than might otherwise be necessary. (Lesser & Hughes, Geriatrics, 2006)
Discuss Behavioral or Psychiatric Conditions with Your Loved
One’s Physicians
First, it's very important
that families know to discuss these issues with physicians. Often, physicians ask about memory & abilities but tend to
not ask about which impairments were abnormalities of behavior.
Many medications have been
tried in attempts to help treat these problems. However, success isn't common & research has shown that complications
from some of the medications may be more serious than the problems for which the medications are being used.
Therefore, if medications
are prescribed, it's very important that families ask about potential side effects so that they can be aware if such problems
occur.
|
 |
|
Mens' minds decline more with age
Trend holds true for cognitive tests, but real-world brain power
likely varies
May 21, 2007
Everyone becomes a little
more forgetful as they get older, but men's minds decline more than women's, according to the results of a worldwide survey.
Certain differences seem to
be inherent in male & female brains:
- Men are better at maintaining & manipulating mental images
(useful in mathematical reasoning & spatial skills)
- Women tend to excel at retrieving information from their brain's
files (helpful with language skills & remembering the locations of objects).
Many studies have looked for
a connection between gender & the amount of mental decline people experience as they age, but the results have been mixed.
Some studies found more age-related
decline in men than in women, while others saw the reverse or even no relationship at all between sex & mental decline.
Those results could be biased because the studies involved older people & women live longer than men: The men tested are
the survivors, "so they're the ones that may not have shown such cognitive decline," said study team leader Elizabeth Maylor
of the University of Warwick in England.
The new study used data from
the BBC Sex ID Internet Survey, conducted between February and May 2005. The survey had more than 250,000 respondents worldwide.
Survey participants completed
4 tasks that tested sex-related cognitive skills:
- matching an object to its rotated form
- matching lines shown from the same angle
- typing as many words in a particular category
as possible in the given time (e.g. "object usually colored grey")
- recalling the location of objects in a line drawing.
The first 2 were tasks at
which men usually excel; the latter are typically dominated by women.
Within each age group studied,
men & women performed better in their respective categories on average. And though performance declined with age for both
genders, women showed significantly less decline than men overall. Women slowed down more in terms of their decline, but when
comparing men & women of the same age, men showed a greater amount of decline.
Maylor cautions that the skills
tested have little practical use & that other factors such as social involvement & mental & physical exercise
have more impact on cognitive decline. So while the trend might hold, there'd definitely be a lot of variation man by man
& woman by woman.
"You can't sort of say, 'Oh,
you're a man, so you're going to decline faster than me because I'm a woman,'" Maylor said.
source: MSNBC.msn
|
 |
 |
 |
|
|
 |
 |
 |
|
|
|

